Abstract
Background:
Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) relapse after allogeneic hematopoietic cell transplantation (HCT) is difficult to manage. One strategy to prevent this is tyrosine kinase inhibitor (TKI) maintenance post-HCT, but the vast majority of data discusses the use of imatinib in this setting, with few reports on newer generation TKIs (NG-TKI). Here, we present our institutional experience with TKI maintenance post-HCT.
Methods:
Ph+ ALL patients who underwent HCT and received TKI maintenance anytime post-HCT from 2003-2019 were included through retrospective chart review. The primary endpoint was overall survival (OS), and secondary endpoints were progression-free survival (PFS), and cumulative incidence of non-relapse mortality (NRM), cumulative incidence of relapse (CIR), and graft-vs-host disease (GVHD). OS and PFS were calculated via the Kaplan Meier method, NRM, CIR, and GVHD were calculated through a competing risk analysis, where CIR was used as a competing risk for NRM and GVHD, and NRM for CIR and GVHD.
Results:
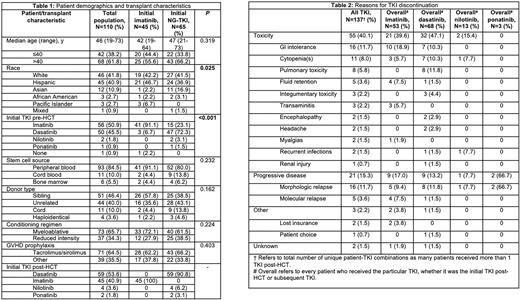
110 patients were included, and their baseline characteristics are described in Table 1. 45 (40.9%) patients received imatinib initially, and 65 (59.1%) received NG-TKI initially, of whom, 59 (90.8%) received dasatinib, 4 (6.2%) received nilotinib, and 2 (3.1%) received ponatinib. The median age (range) of the initial imatinib and NG-TKI groups were 42 years (19-64) and 47 (21-73), respectively. 25 (55.6%) vs 43 (66.2%) in the initial imatinib vs NG-TKI groups were >40 years, respectively. 18 (40.0%) vs 39 (60.0%) of the patients receiving initial imatinib vs NG-TKI were female (P=0.052). 20 (44.4%) vs 54 (83.1%) patients in the imatinib vs NG-TKI group underwent HCT ≥2010, respectively (P<0.001). 41 (91.1%) vs 57 (87.7%) in the imatinib vs NG-TKI groups underwent HCT in first complete remission (CR1) (P=0.76), and 21 (46.7%) vs 31 (47.7%) had no measurable residual disease (MRD) detected by PCR at the time of HCT, although 10 (22.2%) vs 10 (15.4%) did not have a PCR report at the time of HCT available (P=0.66). 42 patients (38.2%) required a dose reduction due to toxicity, and 67 patients (60.9%) experienced TKI interruption due to toxicity. Initial imatinib vs NG-TKI maintenance was discontinued in 20 (46.5%) vs 29 (44.6%) due to toxicity, respectively. The median cumulative initial TKI exposure in patients initially receiving imatinib and NG-TKI was 354 days (range, 6-4,473) and 301 days (range, 5-2,740), respectively. Reasons for TKI discontinuation are listed in Table 2. There was no difference in 5-year OS and PFS in patients receiving imatinib vs NG-TKI, 84.1% vs 69.8% (P=0.47) and 75.1% vs 62.5% (P=0.48), respectively. Likewise, 5-year NRM, 5-year relapse, and 4-year GVHD were similar between imatinib vs NG-TKI, 9.0% vs 18.3% (P=0.48), 15.9% vs 19.2% (P=0.92), and 40.7% vs 47.7% (P=0.47), respectively. Univariate analyses showed an improvement in OS and PFS in those who received ≥12 months of TKI maintenance vs those who received <12 months, hazard ratio (HR) 0.26 (95% confidence interval (CI), 0.12-0.55), P<0.001 and HR 0.33 (95% CI, 0.17-0.64), P<0.001. There was no difference in OS and PFS for those who completed ≥12 months of TKI maintenance based on initial TKI of choice, HR 0.85 (95% CI, 0.21-3.51), P=0.8 and HR 0.91 (95% CI, 0.28-2.94), P=0.9.
Conclusions:
Our findings demonstrate the challenges of delivering post-HCT TKI maintenance. Toxicity leading to TKI interruptions, discontinuation, and dose reduction was common. Post-transplant outcomes were comparable with imatinib and NG-TKI. Therefore, TKI selection for post-HCT maintenance should be based on patient tolerability to maximize TKI maintenance exposure.
Disclosures
Koller:Treadwell Therapeutics: Other: Safety Review Committee; Takeda: Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Al Malki:NexImmune: Consultancy, Research Funding; Hasna Biopharma: Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotec: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; CareDx: Consultancy, Research Funding; Gilead: Consultancy, Research Funding. Salhotra:BMS: Research Funding; Orca Bio: Research Funding; Kadmon: Other: Advisory board meeting . Ali:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Aribi:SeaGen: Consultancy. Ball:Oncovalent: Membership on an entity's Board of Directors or advisory committees. Artz:Abbvie: Honoraria; Magenta: Honoraria. Marcucci:Abbvie: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings. Stein:Amgen: Speakers Bureau. Nakamura:Helocyte Inc: Research Funding; Magenta Therapeutics: Consultancy; Sanofi: Consultancy; Omeros: Consultancy; Kadmon: Consultancy; BluebirdBio: Consultancy. Pullarkat:Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member; AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Aldoss:Kite: Consultancy; Autolus Limited: Consultancy; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Amgen: Consultancy; Agios: Consultancy, Honoraria; AbbVie: Consultancy, Research Funding. Mei:Novartis: Consultancy; Morphosys: Research Funding, Speakers Bureau; EUSA: Honoraria; Celgene: Research Funding; Beigene: Research Funding; Incyte: Research Funding; CTI: Honoraria.
OffLabel Disclosure:
Nilotinib in Ph+ ALL is an off label use
Author notes
Asterisk with author names denotes non-ASH members.